Validation
No worries about internal resources or lack of quality:
With QuACX on your side, all applicable GxP regulations are respected in your computerized system.
Validation
of computerized systems
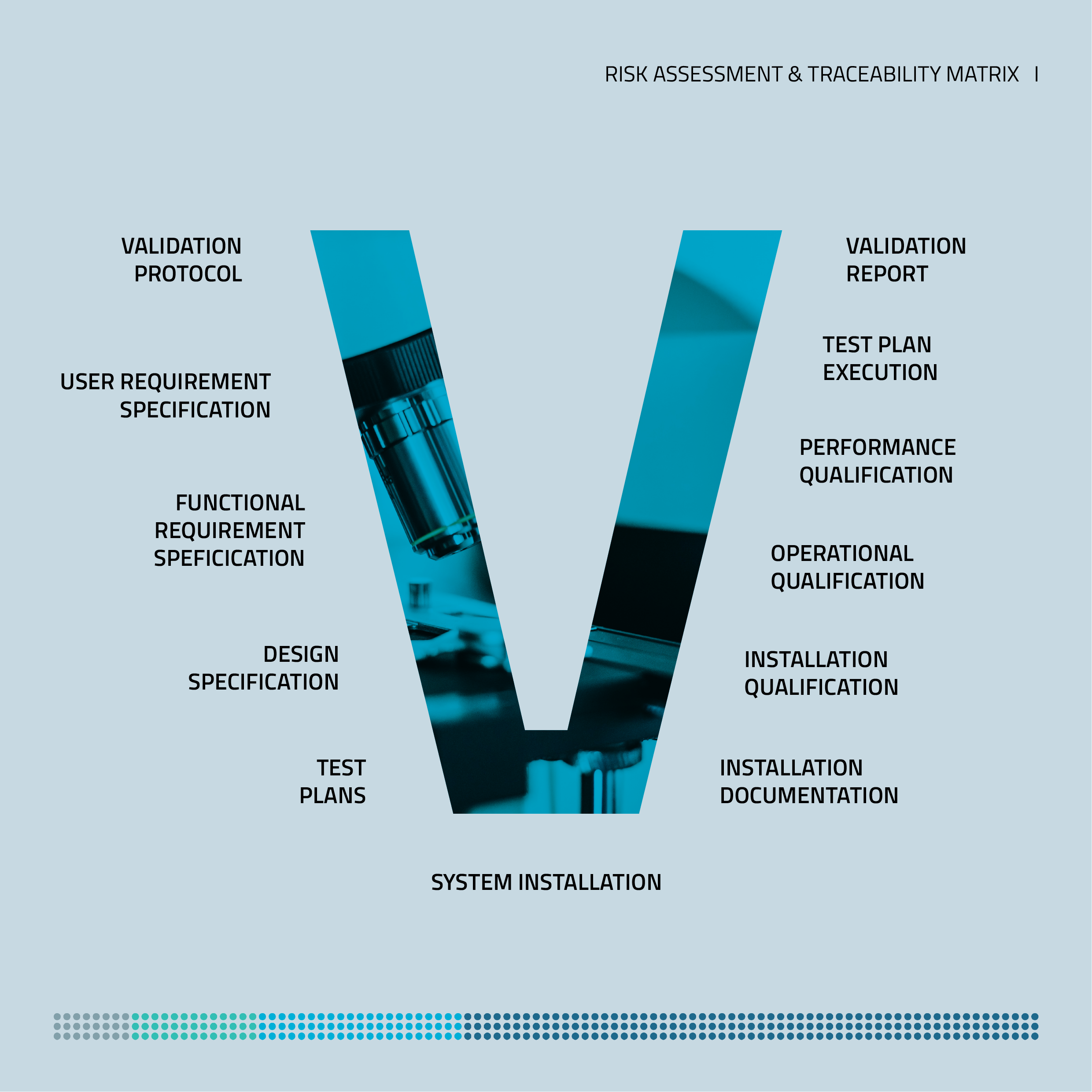
Our validation documentation is carried out according to the V-model
CSV validations and our experience:
As a partner of the manufacturers, QuACX GmbH carries out validations of configurations in the pharmaceutical industry. With more than ten years of experience, our employees have already successfully accompanied several national as well as international audits.
Thanks to this experience, we have collected many recommendations and assistance for the correct procedure, which we would like to provide you with:
